Workshop of alexey pozdnyakov knives and handmade blades. Tungsten Steel Tungsten Carbon Steel
Chemistry
Element 74, tungsten, is usually ranked among the rare metals: its content in the earth's crust is estimated at 0.0055%; it is not found in seawater, it has not been detected in the solar spectrum. However, in popularity, it can compete with many not rare metals, and its minerals were known long before the discovery of the element itself. So, back in the 17th century. in many European countries they knew "tungsten" and "tungsten" - that is how the most common minerals of tungsten were called at that time - wolframite and scheelite. And elementary tungsten was discovered in the last quarter of the 18th century.
Tungsten ore
Very soon, this metal gained practical importance - as an alloying addition. And after the 1900 World Exhibition in Paris, at which samples of high-speed tungsten steel were demonstrated, element No. 74 was used by metallurgists in all more or less industrialized countries. The main feature of tungsten as an alloying additive is that it gives steel a red resistance - it allows you to maintain hardness and strength at high temperatures. Moreover, most steels, when cooled in air (after holding at a temperature close to the temperature of red heat), lose their hardness. And tungsten - no.
Made from tungsten steel, tools withstand the tremendous speeds of the most intense metalworking processes. Cutting speed with such a tool is measured in tens of meters per second.
Modern high speed steels contain up to 18% tungsten (or tungsten with molybdenum), 2-7% chromium and a small amount of cobalt. They retain their hardness at 700-800 ° C, while ordinary steel begins to soften when heated to only 200 ° C. Stellites - alloys
tungsten and with chromium and cobalt (without iron) and especially tungsten carbides - its compounds with carbon. Alloy "visible" (tungsten carbide, 5-15% cobalt and a small admixture of titanium carbide) is 1.3 times harder than ordinary tungsten steel and retains hardness up to 1000-1100 ° C. Cutters from this alloy can be removed in a minute up to 1500-2000 m of iron shavings. They can quickly and accurately process "capricious" materials: bronze and porcelain, glass and ebonite; however, the tool itself wears out very little.
At the beginning of the XX century. tungsten filament began to be used in electric bulbs: it allows you to bring the heat up to 2200 ° C and has a high luminous efficiency. And in this capacity, tungsten is absolutely irreplaceable to this day. Obviously, this is why the electric light bulb is called in one popular song "a tungsten eye".
Minerals and tungsten ores
Tungsten occurs in nature mainly in the form of oxidized complex compounds formed by tungsten trioxide WO 3 and oxides of iron and manganese or calcium, and sometimes lead, copper, thorium and rare earth elements. The most common mineral, wolframite, is a solid solution of tungstates (tungstic acid salts) of iron and manganese (mFeW0 4 * nMnW0 4). This solution is heavy and hard crystals of brown or black color, depending on which compound prevails in their composition. If there is more pobnerite (manganese compounds), the crystals are black, but if iron-containing ferberite predominates, they are brown. Wolframite is paramagnetic and conducts electricity well.
Of the other minerals of tungsten, scheelite - calcium tungstate CaW04 - is of industrial importance. It forms brilliant, like glass, crystals of light yellow, sometimes almost white... Scheelite is non-magnetic, but it has another characteristic feature - the ability to luminescence. When illuminated with ultraviolet rays, it fluoresces in a bright blue color in the dark. An admixture of molybdenum changes the color of the scheelite glow: it becomes pale blue, and sometimes even cream. This property of scheelite, used in geological exploration, serves as a prospecting indicator for finding mineral deposits.
Deposits of tungsten ores are theologically associated with areas of distribution of granites. The largest foreign deposits of wolframite and scheelite are located in China, Burma, USA, Bolivia and Portugal. Our country also has significant reserves of tungsten minerals, their main deposits are located in the Urals, the Caucasus and Transbaikalia.
Large crystals of wolframite or scheelite are very rare. Usually, tungsten minerals are only interspersed into ancient granite rocks - the average concentration of tungsten in the end turns out to be 1-2% at best. Therefore, it is very difficult to extract tungsten from ores.
How tungsten is obtained
The first stage is ore beneficiation, separation of valuable components from the main mass - waste rock. Beneficiation methods are common for heavy ores and metals: grinding and flotation with subsequent operations - magnetic separation (for tungstenite ores) and oxidative roasting.
The resulting concentrate is most often sintered with an excess of soda to convert tungsten into a soluble compound - sodium tungstate. Another way to obtain this substance is by leaching; tungsten is extracted with a soda solution under pressure and at elevated temperatures (the process is in an autoclave), followed by neutralization and precipitation in the form of artificial scheelite, i.e. calcium tungstate. The desire to obtain precisely tungstate is explained by the fact that it is relatively simple from it, in just two stages:
CaW0 4 → H 2 W0 4 or (NH 4) 2 W0 4 → WO 3, tungsten oxide purified from most of the impurities can be isolated.
There is another way to obtain tungsten oxide - through chlorides. The tungsten concentrate is treated with gaseous chlorine at an elevated temperature. The formed tungsten chlorides are quite easily separated from the chlorides of other metals by sublimation, using the temperature difference at which these substances pass into the vapor state. The obtained tungsten chlorides can be converted into oxide, or they can be sent directly for processing into an elemental metal.
The transformation of oxides or chlorides into metal is the next step in the production of tungsten. The best reducing agent for tungsten oxide is hydrogen. Reduction with hydrogen produces the purest metallic tungsten. The reduction process takes place in tube furnaces, heated in such a way that as it moves along the pipe, the "boat" with W0 3 passes through several temperature zones. A stream of dry hydrogen flows towards it. Recovery occurs both in “cold” (450-600 ° C) and “hot” (750-1100 ° C) zones; in the "cold" ones - to the lowest oxide W0 2, then - to the elemental metal. Depending on the temperature and duration of the reaction in the "hot" zone, the purity and size of the grains of powdery tungsten released on the walls of the "boat" change.
The recovery can take place not only under the influence of hydrogen. In practice, coal is often used. The use of a solid reducing agent somewhat simplifies production, but in this case a higher temperature is required - up to 1300-1400 ° C. In addition, coal and the impurities that it always contains react with tungsten, forming carbides and other compounds. This leads to contamination of the metal. Meanwhile, electrical engineering needs very pure tungsten. Only 0.1% iron makes tungsten brittle and unsuitable for making the finest wire.
The production of tungsten from chlorides is based on the pyrolysis process. Tungsten forms several compounds with chlorine. With the help of an excess of chlorine, all of them can be converted into the higher chloride - WCl 6, which decomposes into tungsten and chlorine at 1600 ° С.In the presence of hydrogen, this process takes place already at 1000 ° С.
This is how metallic tungsten is obtained, but not compact, but in the form of a powder, which is then pressed in a stream of hydrogen at a high temperature. At the first stage of pressing (when heated to 1100-1300 ° C), a porous brittle ingot is formed. Pressing continues at an even higher temperature, almost reaching the end of the melting point of tungsten. Under these conditions, the metal gradually becomes solid, acquires a fibrous structure, and with it plasticity and ductility.
Main properties
Tungsten differs from all other metals in its special weight, hardness and refractoriness. The expression has long been known: "Heavy as lead." It would be more correct to say: "Heavy as tungsten." The density of tungsten is almost twice that of lead, more precisely - 1.7 times. Moreover, its atomic mass is slightly lower: 184 against 207.
In terms of refractoriness and hardness, tungsten and its alloys occupy the highest places among metals. Technically pure tungsten melts at 3410 ° C, and boils only at 6690 ° C. Such a temperature is on the surface of the Sun!
And the "king of refractoriness" looks pretty ordinary. The color of tungsten depends to a large extent on the method of production. Fused tungsten is a lustrous gray metal that most closely resembles platinum. Tungsten powder - gray, dark gray and even black (the finer the grain, the darker).
Chemical activity
Natural tungsten consists of five stable isotopes with mass numbers from 180 to 186. In addition, nuclear reactors as a result of various nuclear reactions, 8 more radioactive isotopes of tungsten with mass numbers from 176 to 188 are formed; they are all relatively short-lived, with half-lives ranging from a few hours to several months.
The seventy-four electrons of a tungsten atom are arranged around the nucleus in such a way that six of them are in outer orbits and can be separated relatively easily. Therefore, the maximum valence of tungsten is six. However, the structure of these external orbits is special - they consist, as it were, of two "tiers": four electrons belong to the penultimate level -d, which is thus less than half full. (It is known that the number of electrons in the filled level d is equal to ten.) These four electrons (apparently non-sparse) are capable of easily forming a chemical bond. As for the two "outermost" electrons, it is quite easy to tear them off.
It is precisely the structural features of the electron shell that explain the high chemical activity of tungsten. In compounds, it is not only hexavalent, but also five-, four-, three-, two- and zero-valent. (Only univalent tungsten compounds are unknown).
The activity of tungsten is manifested in the fact that it reacts with an overwhelming amount of elements, forming many simple and complex compounds. Even in alloys, tungsten is often chemically bonded. And it interacts with oxygen and other oxidants more easily than most heavy metals.
The reaction of tungsten with oxygen occurs when heated, especially easily in the presence of water vapor. If tungsten is heated in air, then at 400-500 ° C a stable lower oxide W0 2 is formed on the metal surface; the entire surface is covered with a brown film. At a higher temperature, an intermediate oxide W 4 O 11 of blue color is obtained first, and then lemon-yellow tungsten trioxide W0 3, which sublimes at 923 ° C.
Dry fluorine combines with finely ground tungsten even with slight heating. In this case, hexafluoride WF6 is formed - a substance that melts at 2.5 ° C and boils at 19.5 ° C. A similar compound - WCl 6 - is obtained by reaction with chlorine, but only at 600 ° C. Steel blue crystals of WCl 6 melt at 275 ° C and boil at 347 ° C. With bromine and iodine, tungsten forms unstable compounds: penta- and dibromide, tetra- and diiodin.
At high temperatures, tungsten combines with sulfur, selenium and tellurium, with nitrogen and boron, with carbon and silicon. Some of these compounds are very hard and have other remarkable properties.
The carbonyl W (CO) 6 is very interesting. Here, tungsten is combined with carbon monoxide and therefore has zero valence. Tungsten carbonyl is unstable; it is received in special conditions... At 0 ° C it separates from the corresponding solution in the form of colorless crystals, at 50 ° C it sublimes, and at 100 ° C it completely decomposes. But it is precisely this compound that makes it possible to obtain thin and dense coatings of pure tungsten.
Not only tungsten itself, but also many of its compounds are very active. In particular, tungsten oxide WO 3 is polymerizable. As a result, the so-called isopoly compounds and heteropoly compounds are formed: the molecules of the latter can contain more than 50 atoms.
Alloys
Tungsten forms alloys with almost all metals, but they are not so easy to obtain. The fact is that the generally accepted methods of fusion in this case, as a rule, are not applicable. At the melting point of tungsten, most other metals are already converted into gases or very volatile liquids. Therefore, alloys containing tungsten are usually obtained by powder metallurgy methods.
To avoid oxidation, all operations are carried out in a vacuum or in an argon atmosphere. This is how it is done. First, a mixture of metal powders is pressed, then sintered and arc-melted in electric furnaces. Sometimes one tungsten powder is pressed and sintered, and the porous workpiece obtained in this way is impregnated with a liquid melt of another metal: so-called pseudo-alloys are obtained. This method is used when it is necessary to obtain an alloy of tungsten with copper and silver.
With chromium and molybdenum, niobium and tantalum, tungsten gives conventional (homogeneous) alloys at any ratio. Even small additions of tungsten increase the hardness of these metals and their resistance to oxidation.
Alloys with iron, nickel and cobalt are more complex. Here, depending on the ratio of the components, either solid solutions or intermetallic compounds (chemical compounds of metals) are formed, and in the presence of carbon (which is always present in steel), mixed tungsten and iron carbides, which give the metal even greater hardness.
Very complex compounds are formed when tungsten is fused with aluminum, beryllium and titanium: there are from 2 to 12 light metal atoms per tungsten atom. These alloys are characterized by high temperature resistance and oxidation resistance at high temperatures.
In practice, tungsten alloys are most often used not with any one metal, but with several. These are, in particular, acid-resistant alloys of tungsten with chromium and cobalt or nickel (amala); they are used to make surgical instruments. The best grades of magnetic steel contain tungsten, iron, and cobalt. And in special heat-resistant alloys, in addition to tungsten, there are chromium, nickel and aluminum.
Of all tungsten alloys, tungsten-containing steels have acquired the greatest importance. They are resistant to abrasion, do not crack, and retain their hardness up to the temperature of red heat. The tool of them not only allows to sharply intensify the metalworking processes (the speed of processing metal products increases by 10-15 times), but also lasts much longer than the same tool made of other steel.
Tungsten alloys are not only heat-resistant, but also heat-resistant. They do not corrode at high temperatures under the influence of air, moisture and various chemicals. In particular, 10% of tungsten added to nickel is enough to increase the corrosion resistance of the latter by 12 times! And tungsten carbides with the addition of tantalum and titanium carbides, cemented with cobalt, are resistant to the action of many acids - nitric, sulfuric and hydrochloric - even when boiled. Only a mixture of hydrofluoric and nitric acids is dangerous to them.
The article covers the process of tungsten production from the stage of ore beneficiation to the stage of obtaining billets in the form of bars and ingots. The characteristic features of each stage are noted.
Particular attention in the article is paid to products (wire, rods, sheets, etc.). The processes of manufacturing one or another tungsten product, its characteristic features and fields of application are described.
Chapter 1. Wolfram. Properties and applications of tungsten
Tungsten (denoted by W) - chemical element VI group of the 6th period of the table D.I. Mendeleev, has number 74; transition metal of light gray color. The most refractory metal, has a melting point t pl = 3380 ° C. From the point of view of the use of tungsten metal, its most important properties are density, melting point, electrical resistance, coefficient of linear expansion.§one. Tungsten properties
| Property | Meaning |
|---|---|
| Physical properties | |
| Atomic number | 74 |
| Atomic mass, amu (g / mol) | 183,84 |
| Atomic diameter, nm | 0,274 |
| Density, g / cm 3 | 19,3 |
| Melting point, ° С | 3380 |
| Boiling point, ° С | 5900 |
| Specific heat, J / (g K) | 0,147 |
| Thermal conductivity, W / (m K) | 129 |
| Electrical resistance, μOhm cm | 5,5 |
| Linear thermal expansion coefficient, 10 -6 m / mK | 4,32 |
| Mechanical properties | |
| Young's modulus, GPa | 415,0 |
| Shear modulus, GPa | 151,0 |
| Poisson's ratio | 0,29 |
| Ultimate strength σ B, MPa | 800-1100 |
| Elongation δ,% | 0 |
The metal has a very high boiling point (5900 ° C) and a very low evaporation rate even at a temperature of 2000 ° C. The electrical conductivity of tungsten is almost three times lower than that of copper. The properties that limit the scope of application of tungsten include high density, high tendency to brittleness at low temperatures, low resistance to oxidation at low temperatures.
By appearance tungsten is similar to steel. It is used to create high strength alloys. Tungsten can only be processed (forged, rolled and drawn) when heated. The heating temperature depends on the type of processing. For example, the forging of rods is carried out when the workpiece is heated to 1450-1500 ° C.
§2. Tungsten grades
| Tungsten grade | Characteristics of the brand | The purpose of the additive introduction |
|---|---|---|
| HF | Pure tungsten (no additives) | - |
| VA | Tungsten with silicon alkali and aluminum additives | Increase in the temperature of primary recrystallization, strength after annealing, shape stability at high temperatures |
| VM | Tungsten with silica and thorium additives | An increase in the recrystallization temperature and an increase in the strength of tungsten at high temperatures |
| VT | Tungsten with thorium oxide additive | |
| IN AND | Yttrium oxide doped tungsten | Improving the emissive properties of tungsten |
| Overhead lines | Tungsten with lanthanum oxide additive | Improving the emissive properties of tungsten |
| BP | Tungsten Rhenium Alloy | An increase in the ductility of tungsten after high-temperature treatment, an increase in the temperature of primary recrystallization, strength at high temperatures, electrical resistivity, etc. |
| VRN | Tungsten without additives, in which a high content of impurities is allowed | - |
| MV | Molybdenum and Tungsten Alloys | Increasing the strength of molybdenum while maintaining ductility after annealing |
§3. Tungsten Applications
Tungsten is widely used due to its unique properties. In industry, tungsten is used as a pure metal and in a number of alloys.The main applications of tungsten
1. Special steels
Tungsten is used as one of the main components or alloying element in the production of high-speed steels (contain 9-24% tungsten W), as well as tool steels (0.8-1.2% tungsten W - tungsten tool steels; 2-2.7 % tungsten W - chrome-tungsten-silicon tool steels (also contain chromium Cr and silicon Si); 2-9% tungsten W - chrome-tungsten tool steels (also contain chromium Cr); 0.5-1.6% tungsten W - chrome-tungsten-manganese tool steels (also contain chromium Cr and manganese Mn). Of these steels, drills, cutters, punches, dies, etc. are made. “K” - that the steel is alloyed with molybdenum and cobalt, respectively.Tungsten is also a part of magnetic steels, which are divided into tungsten and tungsten-cobalt.
2. Tungsten carbide-based cemented carbide
Tungsten carbide (WC, W 2 C) is a compound of tungsten with carbon (see). It has high hardness, wear resistance and refractoriness. On its basis, the most productive tool carbide alloys have been created, which contain 85-95% WC and 5-14% Co. Working parts of cutting and drilling tools are made of hard alloys.
3. Heat-resistant and wear-resistant alloys
These alloys use the refractoriness of tungsten. Alloys of tungsten with cobalt and chromium - stellites (3-5% W, 25-35% Cr, 45-65% Co) have become widespread. They are usually applied by surfacing on the surfaces of heavily worn machine parts.
4. Contact alloys and "heavy alloys"
These alloys include tungsten-copper and tungsten-silver alloys. These are quite effective contact materials for the manufacture of working parts of knife switches, switches, electrodes for spot welding, etc.
5. Electrovacuum and electric lighting equipment
Tungsten in the form of wire, tape and various forged parts is used in the production of electric lamps, radio electronics and X-ray technology. Tungsten is the best material for filaments and filaments. Tungsten wire and rods serve as electric heaters for high-temperature furnaces (up to ~ 3000 ° C). Tungsten heaters operate in an atmosphere of hydrogen, inert gas, or vacuum.
6. Welding electrodes
A very important field of application for tungsten is welding. Tungsten is used to make electrodes for arc welding(cm. ). Tungsten electrodes are non-consumable.
Chapter 2. Production of tungsten
§one. The process of obtaining refractory metal tungsten
Tungsten is usually referred to a wide group of rare metals. In addition to this metal, this group includes molybdenum, rubidium and others. Rare metals are characterized by a relatively small scale of production and consumption, as well as a low abundance in the earth's crust. None of the rare metals are directly recovered from raw materials. First, raw materials are processed into chemical compounds. In addition, all rare metal ores undergo additional beneficiation prior to processing.In the process of obtaining a rare metal, three main stages can be distinguished:
- Decomposition of ore material - separation of the recovered metal from the bulk of the processed raw materials and its concentration in a solution or sediment.
- Obtaining pure chemical compounds - isolation and purification of a chemical compound.
- Isolation of metal from the resulting compound - obtaining pure rare metals.
- Tungsten ore beneficiation. It is produced by gravity, flotation, magnetic or electrostatic separation. As a result of enrichment, a tungsten concentrate is obtained containing 55 - 65% of tungsten anhydride (trioxide) WO 3. In tungsten concentrates, the content of impurities is controlled - phosphorus, sulfur, arsenic, tin, copper, antimony and bismuth.
- Obtaining trioxide (anhydride) of tungsten WO 3, which serves as a feedstock for the production of metallic tungsten or its carbide. To do this, it is necessary to perform a number of actions, such as decomposition of concentrates, leaching of an alloy or sinter, obtaining technical tungstic acid, etc. As a result, a product should be obtained containing 99.90 - 99.95% WO 3.
- Getting tungsten powder. Pure metal powder can be obtained from tungsten anhydride WO 3. For this, the anhydride reduction process is carried out with hydrogen or carbon. Reduction with carbon is used less frequently, since in this process WO 3 is saturated with carbides, which makes the metal more brittle and worsens workability. When obtaining tungsten powder, special methods are used to control its chemical composition, grain size and shape, and granulometric composition. For example, a rapid rise in temperature, a low rate of hydrogen supply contribute to an increase in the particle size of the powder.
- Obtaining compact tungsten. Compact tungsten, usually in the form of bars or ingots, is a blank for the production of semi-finished products such as wire, bar, strip, and so on.
§2. Getting compact tungsten
There are two ways to obtain compact tungsten. The first is the application of powder metallurgy methods. The second is by melting in electric arc furnaces with a consumable electrode.Powder Metallurgy Methods
This method of producing malleable tungsten is the most common, since it allows a more even distribution of additives that give tungsten special properties (heat resistance, emission properties, and others).
The process of obtaining compact tungsten by this method consists of several stages:
- pressing of metal powder bars;
- low-temperature (preliminary) sintering of workpieces;
- sintering (welding) of workpieces;
- processing of workpieces in order to obtain semi-finished products - tungsten wire, tape, tungsten rods; usually workpieces are processed under pressure (forging) or subjected to machining cutting (e.g. grinding, polishing).
Using the described method of powder metallurgy, tungsten sticks with a square cross section from 8x8 to 40x40 mm and a length of 280-650 mm are obtained. They have good strength at room temperature, but are very fragile. It should be noted that strength and fragility (the opposite property is plasticity) belong to different groups of properties. Strength is a mechanical property of a material, plasticity is a technological one. Ductility determines the suitability of a material for forging. If the material is difficult to forge, then it is brittle. To improve ductility, tungsten rods are hot forged.
However, the method described above cannot be used to produce large-sized workpieces with a large mass, which is a significant limitation. To obtain large-sized workpieces, the mass of which reaches several hundred kilograms, hydrostatic pressing is used. This method allows to obtain billets of cylindrical and rectangular cross-section, pipes and other products of complex shape. Moreover, they have a uniform density, do not contain cracks and other defects.
Fuse
Smelting is used to obtain compact tungsten in the form of large-sized billets (from 200 to 3000 kg), intended for rolling, pipe drawing, production of products by casting. Melting is carried out in electric arc furnaces with a consumable electrode and / or electron beam melting.
In arc melting, packages of sintered rods or sintered blanks of hydrostatic pressing are used as electrodes. Melting is carried out in a vacuum or a rarefied atmosphere of hydrogen. The result is tungsten ingots. Tungsten ingots have a coarse-crystalline structure and increased brittleness, which is caused by a high content of impurities.
To reduce the content of impurities, tungsten is initially melted in an electron beam furnace. But after this type of melting, tungsten also has a coarse crystalline structure. Therefore, then, in order to reduce the grain size, the resulting ingots are melted in an electric arc furnace, adding small amounts of zirconium or niobium carbides, as well as alloying elements to impart special properties.
To obtain fine-grained tungsten ingots, as well as to manufacture parts by casting, arc skull melting with metal casting into a mold is used.
Chapter 3. Products from tungsten. Bars, wire, strips, powder
§one. Tungsten rods
ProductionTungsten rods are one of the most common products made from tungsten refractory metal. Source material for the production of bars is a bar.
To obtain tungsten rods, the bar is subjected to forging on a rotary forging machine. Forging is carried out in a heated state, since tungsten is very brittle at room temperature. There are several stages of forging. At each subsequent stage, rods of a smaller diameter are obtained than at the previous one.
At the first forging, tungsten rods with a diameter of up to 7 mm can be obtained (provided that the rod has a side length of 10-15 cm). Forging is carried out at a billet temperature of 1450-1500 ° C. Molybdenum is commonly used as the heater material. After the second forging, rods with a diameter of up to 4.5 mm are obtained. It is produced at a bar temperature of 1300-1250 ° C. Further forging produces tungsten rods up to 2.75 mm in diameter. It should be noted that tungsten rods of VT, VL and VI grades are obtained at a higher temperature than rods of VA and VCh grades.
If tungsten ingots, which are obtained by melting, are used as the initial billet, then hot forging is not carried out. This is due to the fact that these ingots have a coarse, coarse-grained structure, and their hot forging can lead to cracking and destruction.
In this case, the tungsten ingots are subjected to double hot pressing (the degree of deformation is about 90%). The first pressing is carried out at a temperature of 1800-1900 ° C, the second - 1350-1500 ° C. The workpieces are then hot forged to produce tungsten rods.
Application
Tungsten rods are widely used in various industries. One of the most common uses is for non-consumable welding electrodes. For such purposes, tungsten rods of the VT, VI, VL brands are suitable. Also tungsten rods of VA, VR, MV brands are used as heaters. Tungsten heaters operate in furnaces up to 3000 ° C in an atmosphere of hydrogen, inert gas or in vacuum. Tungsten rods can serve as cathodes for radio tubes, electronic and gas-discharge devices.
§2. Tungsten electrodes
Arc weldingWelding electrodes are one of the most important components required for welding. They are most widely used in arc welding. It belongs to the thermal class of welding, in which melting is carried out by thermal energy. Arc welding (manual, semi-automatic and automatic) is the most common technological process welding. Thermal energy is created by a volt arc that burns between the electrode and the workpiece (part, workpiece). An arc is a powerful stable electrical discharge in an ionized atmosphere of gases, metal vapors. The electrode delivers an electric current to the weld to create an arc.
Welding electrodes
The welding electrode is a coated (or uncoated) wire rod. There are a wide variety of welding electrodes. They differ in chemical composition, length, diameter, a certain type of electrode is suitable for welding certain metals and alloys, etc. etc. The division of welding electrodes into consumable and non-consumable is one of the most important types of their classification.
Consumable welding electrodes are melted during the welding process, their metal, together with the molten metal of the workpiece being welded, is used to replenish the weld pool. Such electrodes are made of steel and copper.
Non-consumable electrodes do not melt during welding. This type includes carbon and tungsten electrodes. When welding using non-consumable tungsten electrodes, a filler material (usually a welding wire or rod) must be supplied, which melts and, together with the molten material of the workpiece, forms a weld pool.
Also, electrodes for welding are covered and uncoated. The coating has important role... Its components can provide weld metal of specified composition and properties, stable arc burning, and protection of molten metal from exposure to air. Accordingly, the constituents of the coating can be alloying, stabilizing, gas-forming, slag-forming, deoxidizing, and the coating itself can be acidic, rutile, basic or cellulosic.
Welding Tungsten Electrodes
As noted earlier, tungsten electrodes are non-consumable and are used in welding with filler wire. These electrodes are mainly used for welding non-ferrous metals and their alloys (tungsten electrode with an addition of zirconium), high-alloy steels (tungsten electrode with an addition of thorium EWT), as well as a tungsten electrode is well suited for obtaining a welded seam of increased strength, and the parts to be welded can be of different chemical composition.
Welding using tungsten electrodes in argon is quite common. This environment has a positive effect on the welding process and the quality of the weld. Tungsten electrodes can be made from pure tungsten or contain various additives that improve the quality of the welding process and weld seam. A feature of non-consumable welding electrodes made of pure tungsten (for example, a tungsten electrode of the EHCh brand) is not very good arc ignition.
Arc striking takes place in three stages:
- short circuit of the electrode to the workpiece;
- withdrawal of the electrode to a small distance;
- the occurrence of a stable arc discharge.
Argon arc welding (Arc welding with a non-consumable tungsten electrode in an argon atmosphere) This type of welding has proven itself well in welding non-ferrous metals such as molybdenum, titanium, nickel, as well as high-alloy steels. This is a type of arc welding, where the source of the heat required to create the weld pool is an electric current. In this type of argon-arc welding, the main elements are a tungsten electrode and an inert gas argon. During welding, argon is fed to the tungsten electrode and protects it, the arc zone and the weld pool from the atmospheric gas mixture (nitrogen, hydrogen, carbon dioxide). This protection greatly improves the quality characteristics of the weld, and also protects the welding tungsten electrodes from rapid combustion in air. Argon gas can be used when welding a large number of metals and alloys, as it is inert.
Tungsten Electrode Standards
In Russia, non-consumable tungsten electrodes are manufactured in accordance with the requirements of standards and specifications. Among them: GOST 23949-80“Non-consumable tungsten welding electrodes. Technical conditions”; TU 48-19-27-88“Lanthanum tungsten in the form of rods. Technical conditions ”; TU 48-19-221-83“SVI-1 grade yttrium tungsten rods. Technical conditions ”; TU 48-19-527-83“Non-consumable tungsten welding electrodes EHF and EVL-2. Technical conditions ”.
§3. Tungsten wire
ProductionTungsten wire is one of the most common products made from this refractory metal. The starting material for its manufacture is forged tungsten rods with a diameter of 2.75 mm.
Wire drawing is carried out at a temperature of 1000 ° C at the beginning of the process and 400-600 ° C at the end. In this case, not only the wire is heated, but also the die. Heating is carried out by a gas burner flame or an electric heater.
Wire drawing with a diameter of up to 1.26 mm is carried out on a straight chain drawing mill, within a diameter of 1.25-0.5 mm - on a block mill with a coil diameter of ~ 1000 mm, a diameter of 0.5-0.25 - on single drawing machines ...
As a result of forging and drawing, the structure of the workpiece turns into a fibrous one, which consists of crystal fragments elongated along the processing axis. This structure leads to a dramatic increase in the strength of the tungsten wire.
After drawing, the tungsten wire is coated with graphite grease. The surface of the wire must be cleaned. Cleaning is carried out using annealing, chemical or electrolytic etching, electrolytic polishing. Polishing can increase the mechanical strength of tungsten wire by 20-25%.
Application
Tungsten wire is used for the manufacture of resistance elements in heating furnaces operating in an atmosphere of hydrogen, neutral gas or vacuum at temperatures up to 3000 ° C. Also, tungsten wire is used for the production of thermocouples. For this, a tungsten-rhenium alloy with 5% rhenium and a tungsten-rhenium alloy with 20% rhenium ( BP 5/20).
IN GOST 18903-73“Tungsten wire. Range ”specifies the areas of application of wire of the VA, VM, VRN, VT-7, VT-10, VT-15 grades. Tungsten wire VA, depending on the group, surface and metal condition, diameter, is used for the manufacture of spirals of incandescent lamps and other light sources, spiral cathodes and heaters electronic devices, springs of semiconductor devices, loop heaters, non-helical cathodes, grids, springs of electronic devices. Wire of the VRN brand is used to obtain bushings, traverses and other parts of devices that do not require the use of tungsten with special additives.
§4. Tungsten Powder
Pure tungsten powder serves as a raw material for the production of compact tungsten (see). Tungsten carbide WC, which in appearance is also a powder, is used for the manufacture of hard alloys.Depending on the purpose, tungsten powders are distinguished by the average particle size, a set of grains, and other parameters.
The main impurity in tungsten powders is oxygen (0.05 - 0.3%). Metallic impurities are contained in tungsten powders in very small quantities. Often additives from other metals are introduced into tungsten powders, which improve certain properties of the final product. Aluminum, thorium, lanthanum and others are often used as additives.
Tungsten powder BA, which is used for the manufacture of wire, contains uniformly distributed silicon-alkali and aluminum additives (0.32% K 2 O; 0.45% SiO 2; 0.03% Al 2 O 3), powder from refractory metal tungsten of VT grade - thorium oxide additive (0.7 - 5%), VL - lanthanum oxide additive (~ 1% La 2 O 3), VI - yttrium oxide additive (~ 3% Y 2 O 3), VM - silica and thorium additives ( 0.32% K 2 O; 0.45% SiO 2; 0.25% ThO 2).
§five. Tungsten strips (sheets, tapes, foils, plates)
ProductionAs a rule, flat rolled products from tungsten - sheets, strips, plates, foil - are produced using two operations - flat forging and rolling. Tungsten sticks of various sizes are used as a blank.
First, the tungsten sticks are flat-forged with a pneumatic hammer. Forging is carried out at a temperature of 1500-1700 ° C, which decreases to 1200-1300 ° C with deformation. The forging operation continues until a forging is obtained with a thickness of 8-10 mm (with a cross section of a rod of 25x25 mm) or 4-5 mm (with a cross section of a rod of 12x12 mm).
Then the obtained forgings are rolled on rolling mills. At the beginning of the rolling process, the billets are heated to 1300-1400 ° C, then the temperature is lowered to 1000-1200 ° C. Hot rolling produces tungsten sheets, strips and plates up to 0.6 mm thick. To obtain sheets, strips and plates of a smaller size, cold rolling is carried out. To obtain thin sheets of tungsten with a thickness of up to 0.125 mm and a tape (foil) with a thickness of 0.02-0.03 mm, rolling in packages is used. The package consists of several tungsten strips of equal thickness and thicker molybdenum plates that lie on top of the tungsten strips. Molybdenum plates are more plastic and deform faster than tungsten plates. As a result, during rolling, they become thinner than tungsten strips. After one or more transitions, the molybdenum plates have to be replaced with new ones so that the thickness of the stack remains approximately constant. It should be noted that the purpose of this process is the manufacture of precisely a thin tungsten tape (foil). The molybdenum plates here are consumable, which is necessary for rolling in packages.
Tungsten ingots, which are obtained by melting (see), can also serve as blanks for tungsten tape, plates and sheets. The ingots are pre-pressed. From ingots with a diameter of 70-80 mm, rectangular billets with a thickness of 20-25 mm and a width of 50-60 mm are obtained by pressing. Then the workpieces are deformed on two-roll presses.
Tungsten sheets V-MP
V-MP tungsten sheets are widely used in industry. They are made from tungsten powder of grades PV1 and PV2, containing 99.98% W. V-MP sheets and plates should have a thickness of 0.5-45 mm, cut edges. Sheets can be machined according to customer requirements. GOST 23922-79“Sheets from V-MP grade tungsten. Technical conditions ”.
Application
Due to their high heat resistance, tungsten sheets, like other products made from this refractory metal, are used in extremely high temperatures. Various accessories for high-temperature furnaces are made from tungsten sheets - heat shields, supports and other fastening elements. Tungsten sputtering targets, which are made in the form of plates, are used for thin barrier films for metallization of semiconductor components of integrated circuits. In nuclear power, tungsten sheets are used as shields to attenuate the flow of radioactive radiation.
§6. Tungsten-rhenium alloys
It is worth taking out tungsten-rhenium alloys and products from these alloys in a separate paragraph. Alloys of grades BP5 and BP20 will be considered in more detail here.Alloys of these two metals are heat-resistant. Alloying tungsten with other metals lowers its melting point. But when alloying with a refractory metal, the melting point of the alloy does not decrease so significantly. Tungsten (W) and rhenium (Re) are refractory metals.
When rhenium is used as an additive, a “rhenium effect” is observed. 5% rhenium increases the heat resistance and ductility of tungsten. At 20-30% rhenium content, an optimal combination of strength and ductility with high manufacturability is observed. Also, the advantages of tungsten-rhenium alloys include a low evaporation rate at operating temperatures and a high electrical resistance.
Alloys of tungsten with rhenium, like compact tungsten, are obtained by powder metallurgy and smelting.
An interesting area of application for these alloys is temperature measurement. Tungsten-rhenium wire BP5 (5% Re, the rest is W) and BP20 (20% Re, the rest is W) are used for the manufacture of high-temperature thermocouples.
The main advantage of such thermocouples is the range of measured temperatures. Because the alloys VR 5/20 are heat-resistant, then with the help of thermocouples made of a suitable wire, temperatures above 2000 ° C can be measured. However, this type of thermocouple must be in an inert environment.
Most often for the manufacture of thermocouples, tungsten-rhenium thermoelectrode wire VR5, VR20 Ø 0.2 is used; 0.35; 0.5 mm.
§7. Tungsten carbides
Tungsten-carbon compounds - tungsten carbides - are very important from a practical point of view. Tungsten forms two carbides - W 2 C and WC. These carbides differ in solubility in carbides of other refractory metals and in chemical behavior in various acids. Tungsten carbides, like carbides of other refractory metals, have metallic conductivity and a positive coefficient of electrical resistance. The refractoriness and high hardness of carbides are due to strong interatomic bonds in their crystals. Moreover, the high hardness of WC carbide remains at elevated temperatures.The most common method for producing tungsten carbides WC and W 2 C is calcining a mixture of powdered tungsten with soot in the temperature range 1000-1500 ° C.
Tungsten carbides WC and W 2 C are mainly used for the manufacture of hard alloys.
Hard alloys
There are 2 groups of tungsten carbide-based hard alloys:
- Cast cemented carbides (often referred to as cast tungsten carbides)
- sintered cemented carbides.
Sintered cemented carbide They combine tungsten monocarbide WC and a cementing metal-binder, which is usually cobalt, less often nickel. Such alloys can only be obtained by powder metallurgy. Tungsten carbide powder and cobalt or nickel powder are mixed, pressed into products of the required shape, and then sintered at temperatures close to the melting temperature of the cementing metal. In addition to high hardness and wear resistance, these alloys have good strength. Sintered cemented carbides are the most productive modern metal cutting tool materials. They are also used for the manufacture of dies, dies, drilling tools. Among the hard alloys for the production of which tungsten carbide is used, it is worth highlighting the alloys of the VK group - tungsten-cobalt hard alloys. Widespread in the industry received alloys VK8 and VK6. They are used for making cutters, drills, cutters, as well as other cutting and drilling tools.
Conclusion
This article discusses various aspects associated with the refractory metal TUNGSTEN - properties, applications, production, products.As described in the article, the process of obtaining this metal consists of many stages and is quite laborious. The authors tried to highlight the most significant stages of tungsten production and draw attention to important features.
A review of the properties and fields of application of tungsten shows that it is a very important material, which is simply impossible to do without in some industries. It has unique properties that, in some situations, cannot be obtained by using other materials.
An overview of commercially available tungsten products - wire, rods, sheets, powder - allows you to better understand its features, important properties and specific applications.
Steel where the main alloying element is. It has been used since the beginning of the 20th century. Distinguish between tungsten steel, alloyed only with tungsten, and complex-alloyed tungsten steel, to which, in addition to tungsten, other elements are added. In steel, it is partially in solid solution and forms persistent, sparingly soluble carbides, as a result of which its tendency to grain growth decreases when heated to high tr and irreversible temper brittleness, hardenability and, consequently, strength and toughness increase.
In many tungsten steel alloyed with chromium, metastable carbides of the (W, Cr, Fe) 23 C6 type are formed, which dissolve easily upon heating, which significantly lowers the critical quenching rate and improves hardenability. Tungsten steel is smelted in electric (induction) furnaces, in which good electrodynamic stirring of steel ensures complete dissolution of tungsten. Hard-alloyed tungsten steels are used as structural steels, tool steels, as well as steels with special physical properties. and chem. St. you, for example. heat-resistant steels. Structural V. s. characterized by a low tendency to overheating, fine-grained, increased strength and ductility, they are not prone to temper brittleness. Fur. Holy Islands of these steels are improved by quenching and high-temperature tempering.
From structural tungsten steel grades 18X2N4VA and 15XNG2VA (also used in a carburized state) are made crankshafts, gear wheels and other machine parts operated at high speeds, shock loads and vibrations, rotor disks, parts of compressors and gearboxes, operated at temperatures up to 400 ° C, from 38KhNZVA steel, are used for making heavily loaded parts e.g. crankshafts, along with tungsten, are alloyed with molybdenum. Tool steels of the pearlite class are distinguished by their wear resistance.
The deformation of the tool made of this steel is reduced during hardening. Tool steels of the carbide class are characterized by increased heat resistance due to the formation of secondary high-alloy martensite with high hardness and stability, as well as the precipitation of high-strength dispersed carbides. Billets for instrumental V. s. in front of the fur. by processing they are annealed to granular perlite at temperature 780-800 ° C for softening and better workability. Tool tungsten steel grades KhVSG and KhV4 are quenched from temperature 820-840 ° C in oil heated to temperature 60-80 ° C and tempered at temperature 160-180 ° C. Steel hardness after such heat treatment is 66-67 HRS.
Tool tungsten steels are used to produce cutting tools, dies and rolls for cold and hot rolling. High-temperature steels of the martensitic and austenitic classes, alloyed with tungsten, are used for the manufacture of steam pipelines, disks and turbine blades. Heat treatment of these steels consists of quenching in water from 1000-1150 ° C and subsequent tempering or aging at 600-800 ° C for 2-3 hours. Grades, chem. composition and fur. St.-va constructional V.
Lit .: Geller O. A. Tool steels .; Chemistry and technology of molybdenum and tungsten
You are reading an article on the topic of tungsten steel
The foundry industry generally uses steel molds for aluminum products. Manufacturers have to reduce lead times to stay competitive. Since in such cases the steel is often already reaching its limits, manufacturers have resorted to using tungsten.
Materials scientists at Plansee studied the properties of X37CrMoV5-1 (DIN 1.2343 / ASTM) heat-resistant tool steel and Densimet® tungsten alloy. Tungsten alloy features superior corrosion resistance and thermal conductivity.
Thermal conductivity
The thermal conductivity of tungsten alloy is higher than that of steel, and it remains stable up to 500 ° C. This superior heat dissipation performance provides more short casting cycles.
In addition, the risk of thermal stress, thermal cracking or deformation is reduced. Thanks to this, Densimet ® has a longer service life compared to steel.
Another advantage: faster heat dissipation results in a significantly finer microstructure of the castings and improved mechanical properties. This means to you lower marriage rate, and for your customers - ideal characteristics cast products, including high strength and less porosity.
Corrosion resistance
Densimet® casting inserts are particularly resistant to erosion and corrosion. Traditional casting liners and cores are particularly susceptible to erosion when injected with aluminum at high speed. Since tungsten does not react with molten aluminum, a Densimet® liner can be used four times longer than steel liners.
Detailed results are published in Properties and Possible Improvement of Tungsten Heavy Alloys for Die Casting Application by Rafael Cury and Laurent Dartus. Co-author Johannes Schröder will present these research results in September in Milwaukee, USA, at the Die Casting Congress and Tabletop of the North American Die Casting Association (NADCA).
Die Casting Congress and Tabletop
September 22-24, 2014
Exhibition Center Wisconsin Center in Milwaukee / USA
We manufacture various products from Densimet® tungsten alloys according to customer needs, such as mold inserts, cooling pins or gating sleeves. Densimet® is an alloy of pure tungsten with alloying additions: nickel and iron (Densimet® D185) or molybdenum, nickel and iron (Densimet® D2M). Our experts will be happy to advise you when choosing a material. You will find more detailed information and contact details of our specialists.
 What you need to open a hookah lounge, and how to do it correctly
What you need to open a hookah lounge, and how to do it correctly How to start a business and choose donut equipment
How to start a business and choose donut equipment Opening a company in Montenegro Open a company in Montenegro
Opening a company in Montenegro Open a company in Montenegro The carpentry shop as a business
The carpentry shop as a business How to choose a business direction?
How to choose a business direction?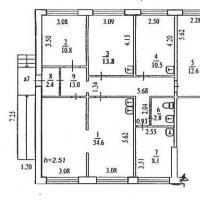 Sample business plan of a dental office
Sample business plan of a dental office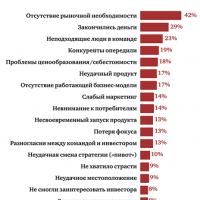 Five best business ideas that brought millions What business to open so as not to go bankrupt
Five best business ideas that brought millions What business to open so as not to go bankrupt